Driving Solutions
Since 1980, IRP Medical has been dedicated to a singular focus; molding Critical-To-Function (CTF) engineered components for the Medical Device Industry.
With ISO 13485 certification and FDA registration, we offer scalable solutions using tested and proven molding and processing technology. Our experienced engineering and tooling group gives us the advantage to go from prototype to millions of parts, allowing our customers to achieve their quality and volume targets.
We are critical, precise, period.
Driving Solutions
Since 1980, IRP Medical has been dedicated to a singular focus; molding Critical-To-Function (CTF) engineered components for the Medical Device Industry.
With ISO 13485 certification and FDA registration, we offer scalable solutions using tested and proven molding and processing technology. Our experienced engineering and tooling group gives us the advantage to go from prototype to millions of parts, allowing our customers to achieve their quality and volume targets.
We are critical, precise, period.
CRITICAL-TO-FUNCTION Engineering

Project Management

Turn-key manufacturing solutions from prototype to mass production of extremely difficult elastomer molded components with very tight tolerance requirements.
Design for manufacturability.
Large scale and time-sensitive projects are met by running manufacturing operations 24/7 around the clock as required.

Tooling
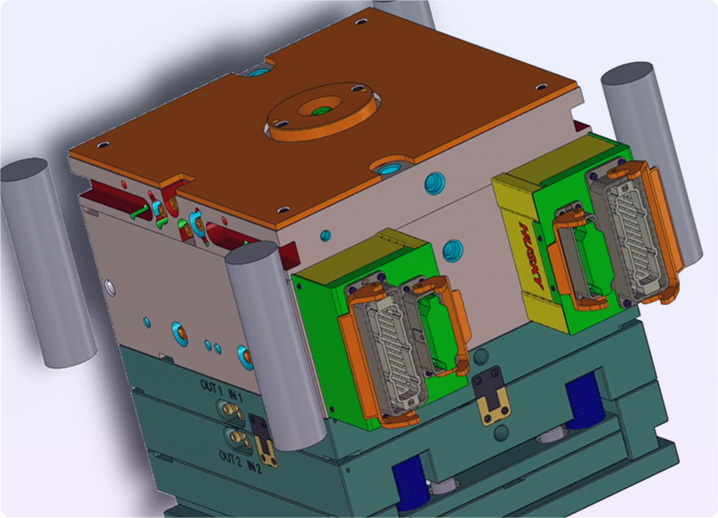
- Our tooling solutions enable us to produce the highest-quality and complex liquid silicone and rubber molded components.
- Cold Runner, fully automated work cells, and semi-automatic hot runner tooling.
- Flashless transfer tooling
Gum Injection
Compression
Customized rubber formulations
Thermoplastic Molding for LSR Overmolding applications

Molding
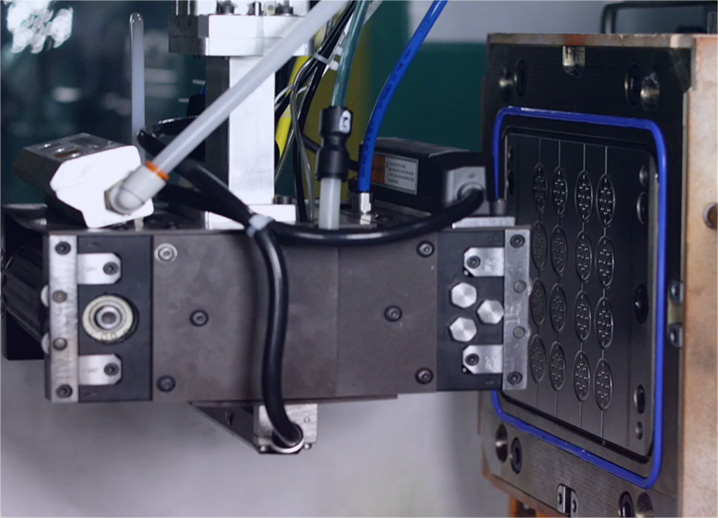
- Since its conception, our tenured engineering team has been developing and refining Liquid Injection Molding (LIM) solutions.
- State-of-the-art, late model LIM Molding machines.
- ISO 7 & 8 Clean Room environments and an ISO 13485:2016 certified facility.
CRITICAL-TO-FUNCTION Engineering

Project Management

Turn-key manufacturing solutions from prototype to mass production of extremely difficult elastomer molded components with very tight tolerance requirements.
Design for manufacturability.
Large scale and time-sensitive projects are met by running manufacturing operations 24/7 around the clock as required.

Tooling

- Our tooling solutions enable us to produce the highest-quality and complex liquid silicone and rubber molded components.
- Cold Runner, fully automated work cells, and semi-automatic hot runner tooling.
- Flashless transfer tooling
Gum Injection
Compression
Customized rubber formulations
Thermoplastic Molding for LSR Overmolding applications

Molding

- Since its conception, our tenured engineering team has been developing and refining Liquid Injection Molding (LIM) solutions.
- State-of-the-art, late model LIM Molding machines.
- ISO 7 & 8 Clean Room environments and an ISO 13485:2016 certified facility.
